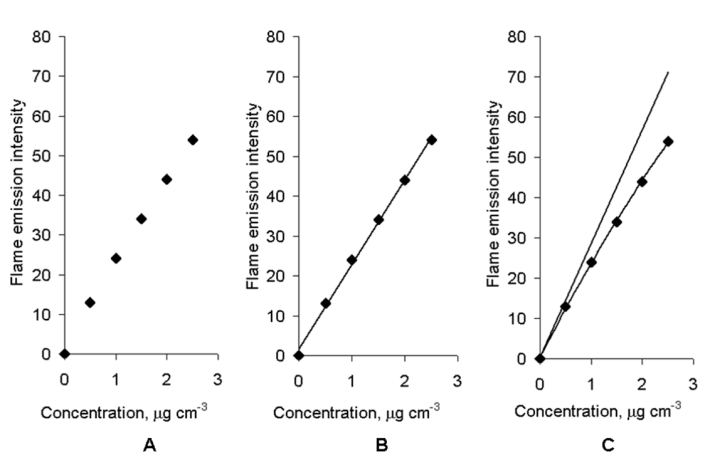
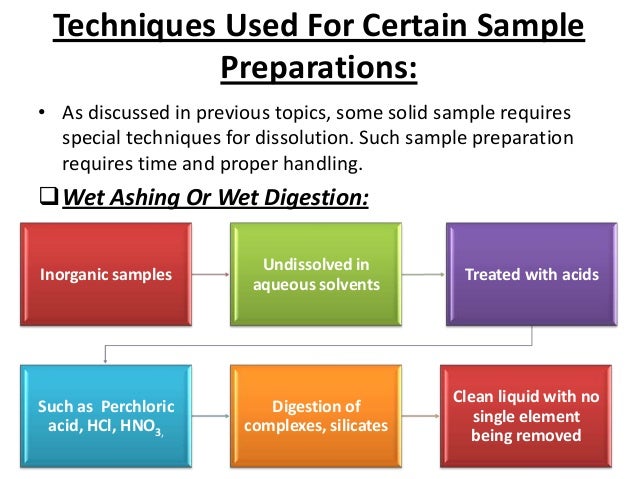
Flame Atomic Absorption Spectroscopy Discussion

Flame atomic absorption spectrometry is a sensitive technique for the quantitative determination of more than sixty metals.
Flame atomic absorption spectroscopy discussion. A flame emission spectroscopy fes. It can also be used to detect if there is trace metals present in food. Principle of atomic absorption emission spectroscopy. A light beam is directed through the flame into a monochromator and onto a detector that measures the amount of light absorbed by the atomized metal in the flame.
Its popularity as compared with that of flame emission is due to its relative freedom from interferences by inter element effect and its relative insensitivity to various in flame temperature. Encyclopedia of spectroscopy and spectrometry third edition 2017. When a small amount of a solution of a metal ion is placed in the flame of a bunsen burner the flame turns a color that is characteristic of the metal ion. We measure the intensity of molecular bands or atomic or ionic lines emitted by excited molecules excited atoms or even by excited ions.
Flame spectrometry includes three methods. Atomic absorption spectroscopy aas and atomic emission spectroscopy aes is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation light by free atoms in the gaseous state atomic absorption spectroscopy is based on absorption of light by free metallic ions. In flame atomic absorption spectrometry a sample is aspirated into a flame and the metals are atomized. B flame atomic absorption spectroscopy faas.
Atomic absorption spectroscopy is one of the most widely used techniques for the determination of metals at trace levels in solution. In analytical chemistry the technique is used for. Flame aas is usually performed on dissolved samples and is a simple very rapid and generally robust interference free technique for analysis of selected elements with simple external standardization with matrix matched solutions. Current research flame atomic absorption spectrometric determination of trace amounts of silver after solid phase extraction with 2mercaptobenzothiazole immobilized on microcrystalline naphthalene a simple and sensitive solid phase extraction procedure combined with flame atomic was designed for the extraction and determination of trace amounts of silver absorption spectrometry.
As it is used for determining the concentration of metals it can be applied in environmental analysis. Copper is analyzed at a wavelength of 324 8 nm with a slit width of 0 5 nm and zinc is analyzed at 213 9 nm with a slit width of 1 0 nm. Learn how to operate the perkin elmer 2280 atomic absorption aa spectrophotometer. Flame atomic absorption spectroscopy.